News | TCTAP 2023
The MitraClip Shows Promising Results in 5-Year COAPT Trial for Heart Failure Patients
Mitral & Tricuspid Valve Therapy

Gregg W. Stone, MD
Mount Sinai Healthcare System in New York City, USA
Gregg W. Stone, MD (Mount Sinai Healthcare System in New York City, USA) discussed the final 5-year results of the COAPT trial, which examined the effectiveness of the MitraClip in patients with secondary mitral regurgitation (MR) and heart failure on May 8th at TCTAP 2023.
Considered the largest randomized trial to date for patients with MR, the COAPT trial involved 614 heart failure patients who were already receiving guideline-directed medical therapy (GDMT). These patients experienced moderate to severe MR, leading to left ventricular dilatation and mitral leaflet tethering caused by papillary muscle displacement. The trial aimed to determine whether addressing the underlying abnormal left ventricle would improve patient outcomes.
The participants were randomly assigned to receive either GDMT alone or treatment with the MitraClip. Earlier publications presented the 2-year results, which revealed a substantial reduction of approximately 50% in heart failure hospitalizations (HFH) among patients receiving medical therapy. The recent 5-year results, published in a NEJM, demonstrated a sustained 50% reduction in HFH. Specifically, the patients treated with GDMT alone experienced 447 hospitalizations (approximately two per patient), while those treated with the MitraClip had 314 HFH in 151 patients, resulting in an average reduction from 57% to 33% per year. This marked a statistically significant overall reduction of 47% in hazards.
The trial also prioritized safety outcomes, employing a composite measure recommended by the FDA that encompassed device-specific events and progressive heart failure necessitating a heart transplant. The results indicated that within the first year, the measure remained below 12%, achieving a rate of 3.3%. Moreover, only four device-related events (1.4%) occurred in the MitraClip group. The MitraClip procedure is well-known for its safety, and this analysis further substantiated its favorable safety profile. Extending the safety assessment to one year, no abnormal safety events related to the MitraClip, such as device detachment, embolization, endocarditis, severe mitral stenosis requiring surgery, or any other device-related complications, were reported in the MitraClip group.
An analysis of the composite outcome of death or HFH demonstrated a significant 47% reduction at the 5-year mark for patients who received the MitraClip in comparison to GDMT alone (Figure 1). Importantly, this reduction remained consistent across all pre-specified subgroups, including age, gender, surgical risk, ventricular size, and other factors analyzed. When examining the time to the first HFH, a Kaplan-Meier analysis revealed a remarkable 51% reduction. Notably, the majority of this reduction occurred within the first three years, after which no significant differences were observed between the two groups.
Regarding all-cause mortality, the trial demonstrated a notable absolute reduction of 10% and a relative reduction of 28% over the 5-year duration. However, a landmark analysis unveiled that the mortality reduction was primarily observed within the first two years, with minimal differences between years two and five. This observation could be attributed to the crossover permitted in the trial, whereby patients in the control group who survived for two years and still exhibited severe MR became eligible for treatment with the MitraClip.
Stone explained, “I think that's a really important secondary implication of this study. Waiting until the patients have been observed for several years is futile because a lot of them are going to die every single year. You can prevent a lot of those deaths and reduce HFH by identifying appropriate patients for the MitraClip treatment despite optimal GDMT as soon as possible.”
In conclusion, the 5-year results of the COAPT trial provided promising evidence regarding the efficacy of the MitraClip in heart failure patients with secondary MR. The findings demonstrate that transcatheter edge-to-edge repair with the MitraClip is safe.
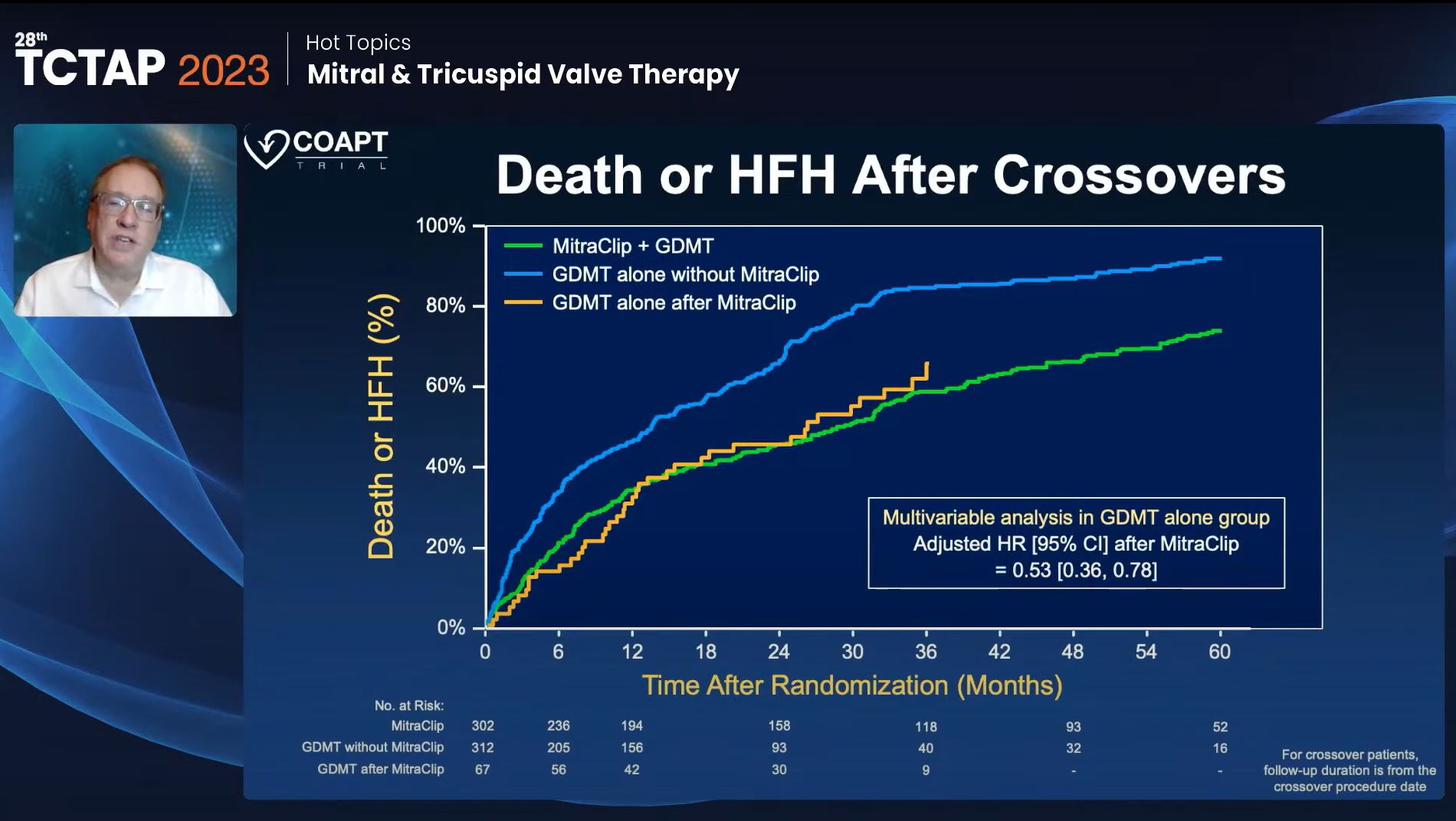
Figure 1. Death or HFH after crossovers
Hot Topics
Mitral & Tricuspid Valve Therapy
Monday, May 8, 11:10 AM - 12:20 AM
Valve & Endovascular Theater, Vista 1, B2
Edited by

Junghoon Lee, MD
Catholic university of Korea, Eunpyeong St. Mary's hospital, Korea (Republic of)

Gregg W. Stone, MD
Mount Sinai Healthcare System in New York City, USA
Gregg W. Stone, MD (Mount Sinai Healthcare System in New York City, USA) discussed the final 5-year results of the COAPT trial, which examined the effectiveness of the MitraClip in patients with secondary mitral regurgitation (MR) and heart failure on May 8th at TCTAP 2023.
Considered the largest randomized trial to date for patients with MR, the COAPT trial involved 614 heart failure patients who were already receiving guideline-directed medical therapy (GDMT). These patients experienced moderate to severe MR, leading to left ventricular dilatation and mitral leaflet tethering caused by papillary muscle displacement. The trial aimed to determine whether addressing the underlying abnormal left ventricle would improve patient outcomes.
The participants were randomly assigned to receive either GDMT alone or treatment with the MitraClip. Earlier publications presented the 2-year results, which revealed a substantial reduction of approximately 50% in heart failure hospitalizations (HFH) among patients receiving medical therapy. The recent 5-year results, published in a NEJM, demonstrated a sustained 50% reduction in HFH. Specifically, the patients treated with GDMT alone experienced 447 hospitalizations (approximately two per patient), while those treated with the MitraClip had 314 HFH in 151 patients, resulting in an average reduction from 57% to 33% per year. This marked a statistically significant overall reduction of 47% in hazards.
The trial also prioritized safety outcomes, employing a composite measure recommended by the FDA that encompassed device-specific events and progressive heart failure necessitating a heart transplant. The results indicated that within the first year, the measure remained below 12%, achieving a rate of 3.3%. Moreover, only four device-related events (1.4%) occurred in the MitraClip group. The MitraClip procedure is well-known for its safety, and this analysis further substantiated its favorable safety profile. Extending the safety assessment to one year, no abnormal safety events related to the MitraClip, such as device detachment, embolization, endocarditis, severe mitral stenosis requiring surgery, or any other device-related complications, were reported in the MitraClip group.
An analysis of the composite outcome of death or HFH demonstrated a significant 47% reduction at the 5-year mark for patients who received the MitraClip in comparison to GDMT alone (Figure 1). Importantly, this reduction remained consistent across all pre-specified subgroups, including age, gender, surgical risk, ventricular size, and other factors analyzed. When examining the time to the first HFH, a Kaplan-Meier analysis revealed a remarkable 51% reduction. Notably, the majority of this reduction occurred within the first three years, after which no significant differences were observed between the two groups.
Regarding all-cause mortality, the trial demonstrated a notable absolute reduction of 10% and a relative reduction of 28% over the 5-year duration. However, a landmark analysis unveiled that the mortality reduction was primarily observed within the first two years, with minimal differences between years two and five. This observation could be attributed to the crossover permitted in the trial, whereby patients in the control group who survived for two years and still exhibited severe MR became eligible for treatment with the MitraClip.
Stone explained, “I think that's a really important secondary implication of this study. Waiting until the patients have been observed for several years is futile because a lot of them are going to die every single year. You can prevent a lot of those deaths and reduce HFH by identifying appropriate patients for the MitraClip treatment despite optimal GDMT as soon as possible.”
In conclusion, the 5-year results of the COAPT trial provided promising evidence regarding the efficacy of the MitraClip in heart failure patients with secondary MR. The findings demonstrate that transcatheter edge-to-edge repair with the MitraClip is safe.

Hot Topics
Mitral & Tricuspid Valve Therapy
Monday, May 8, 11:10 AM - 12:20 AM
Valve & Endovascular Theater, Vista 1, B2
Edited by

Junghoon Lee, MD
Catholic university of Korea, Eunpyeong St. Mary's hospital, Korea (Republic of)


Leave a comment