News | SEOUL VALVES 2022
Not just alternative to SAVR: 'TAVR has become standard of treatment for severe, symptomatic AS'
AMC Heart Institute's Chairman Seung-jung Park, MD, PhD says 'For Tissue Valves, SAVR is Alternative to TAVR' in Severe, Symptomatic AS Patients Over 65 Years
Transcatheter aortic valve replacement (TAVR) is expanding rapidly to younger and lower-risk aortic stenosis (AS) patients, becoming the therapy of choice in select subgroups and trumping even surgical aortic valve replacement (SAVR), an expert said.
 "At Asan Medical Center (AMC), the rule is that TAVR is the standard treatment when tissue valves are needed for symptomatic severe AS patients over 65 years of age," said Seung-jung Park, MD, PhD (AMC, Seoul, South Korea) at AP VALVES & STRUCTURAL HEART 2022 on Aug 11, which ran for two days at the Grand Walkerhill Seoul in South Korea.
"At Asan Medical Center (AMC), the rule is that TAVR is the standard treatment when tissue valves are needed for symptomatic severe AS patients over 65 years of age," said Seung-jung Park, MD, PhD (AMC, Seoul, South Korea) at AP VALVES & STRUCTURAL HEART 2022 on Aug 11, which ran for two days at the Grand Walkerhill Seoul in South Korea.
As chairman of AMC's Heart Institute, Park highlighted TAVR advances worldwide and at the medical institution over the past decade.
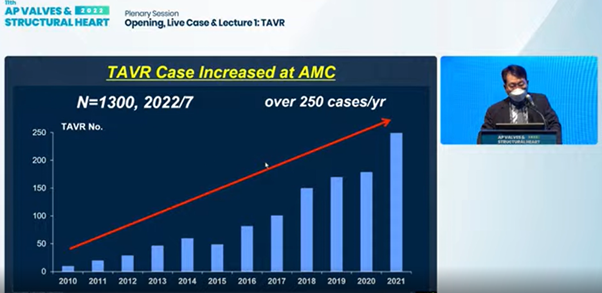
Seung-jung Park, MD, PhD presents on improvements of TAVR worldwide and at Asan Medical Center at the AP VALVES & SH 2022 at the Grand Walkerhill Seoul in South Korea on Aug 11.
Major randomized controlled trials (RCTs) on TAVR and transcatheter heart valves (THVs) - including the CoreValve, Evolut and Sapien valves - laid the groundwork for device approval in both Europe and the US, ushering in the "era of TAVR" for patients who had no alternative to surgery.
TAVR has become comparable with surgery 5 to 8 years since its introduction and SAVR has become the alternative
The landmark PARTNER 31 and the Evolut Low Risk2 trials, published simultaneously in the New England Journal of Medicine (NEJM) in May 2019, backed the case for TAVR in lower operative risk groups after results favored TAVR over SAVR for the 30-day and 1-day endpoints of death, disabling stroke or rehospitalization.
Meta-analysis3 of the NOTION, SURTAVI, PARTNER 3 and Evolut Low Risk trials, published in the Journal of the American College of Cardiology (JACC) in Sep 2019, also showed 1-year outcomes favoring TAVR over SAVR for all-cause mortality (2.1% vs. 3.5%, RR 0.61; 95% CI, 0.39-0.96, I2=0%) and cardiovascular death (1.6% vs. 2.9%, RR 0.55, 0.33-0.90, p=0.02, I2=0%).
TAVR also had lower rates of disabling stroke (0.5 vs 1.7%), atrial fibrillation, life-threatening or disabling bleeding and acute kidney injury but higher rates of permanent pacemaker implantation and moderate or severe paravalvular leak (PVL).
On the heels of the major trials, the US Food and Drug Administration (FDA) greenlighted expanded indications for TAVR, for patients at low surgical risk in Aug 20194, causing seismic shifts in both American and European guidelines and boosting the once experimental procedure to a standard of care.
The 2020 American College of Cardiology and American Heart Association (ACC/AHA) guidelines gave a Class I recommendation for TAVR for patients over 65 years and the 2021 European Society of Congress and European Association of Cardio-Thoracic Surgeons (ESC/EACTS) guidelines greenlighted TAVR with a Class I indication for patients over 75 years5.
"More research is needed for TAVR in bicuspid aortic valve (BAV) disease, aortic and mitral bioprosthetic valve failure, low-flow, low-gradient AS, high-risk aortic regurgitation (AR) and routine use of cerebral protection devices, among others," he said.
"Nevertheless, TAVR has become comparable with surgery 5 to 8 years since its introduction, and SAVR has become the alternative."
AMC Celebrates 97% Procedural Success Rate During 22 Years of TAVR
 Following the first operation at AMC in 2010, - undertaken with TAVR pioneer Alain G. Cribier, MD(University of Rouen's Charles Nicolle Hospital, Paris, France),6 - TAVR operations at the South Korean hospital skyrocketed to over 250 cases per year.
Following the first operation at AMC in 2010, - undertaken with TAVR pioneer Alain G. Cribier, MD(University of Rouen's Charles Nicolle Hospital, Paris, France),6 - TAVR operations at the South Korean hospital skyrocketed to over 250 cases per year.
Last June, AMC's Heart Institute announced becoming the first in Asia to complete 1,000 TAVR cases7 and reported tallying 1,300 cumulative cases this year.
Analysis found AMC's Heart Team achieved a procedural success rate of 96.8% (1,101/1,137 patients) with low rates of new permanent pacemakers (6.9%) and moderate or severe paravalvular leaks (4.3%) despite operating on an elderly, high-risk and severe-disease population.
The rule at AMC, for tissue valves, is that TAVR has become the standard of treatment in patients with symptomatic severe AS patients over 65
Baseline characteristics showed the average patient was 80 years old, 47% were male and the mean Society of Thoracic Surgeons (STS) risk score was 4. Major comorbidities included hypertension (79.1%), diabetes (34.9%), chronic obstructive pulmonary disease (22%), atrial fibrillation (12.4%), stroke (11.7%) and peripheral vascular disease (5.1%).
Comparing 30-day and 1-year outcomes between cases from 2021 and all cumulative cases at AMC showed improvements in the endpoints of all-cause mortality (All cases 1.7% vs. 2021 cases 1.9%), and major, disabling strokes (1.0% vs. 0.3%).
TAVR also recorded improvements for the 30-day and 1-year endpoints of major vascular complications (5.3% vs. 0.0%), new permanent pacemaker implementation (6.9% vs. 3.0%) and moderate or severe PVR (4.3% vs. 1.1%) - which were aided by implementing a unique "minimalist approach."
"Since the first case in 2010, AMC developed a minimalist approach coined the 'MAC' that helps simplify the TAVR procedure," Park said. "Along with impeccable heart team collaboration, we also developed a meticulous pre-TAVR CT measurement algorithm that aids device selection.
The MAC approach implies no general anesthesia, no transesophageal echocardiography (TEE), 30-minute procedures, 1-day stays in the CCU, discharge within 3 days and a cardiac rehabilitation program, Park said.
Outcomes between balloon-expandable and self-expandable valves were also comparable, although self-expandable valves had a significantly higher rate of new permanent pacemakers (5.3% vs. 15.4%).
At AMC, the Edwards Lifesciences’ Sapien 3 valve (73%) accounted for most implants, followed by Sapien XT (Edwards Lifesciences; 10%), Evolut R & PRO (Medtronic; 8%), and CoreValve (Medtronic; 8%).
"Although valve durability remains a major issue and more research is needed on patient subgroups, outcomes at AMC have improved over time, thanks to MAC," Park said. "And the rule here, for tissue valves, is that TAVR has become the standard of treatment in patients with symptomatic severe AS patients over 65."
Edited by

Do-Yoon Kang, MD
Asan Medical Center, Korea (Republic of)
Written by

YoonJee Marian Chu, Medical Journalist
Read Biography
1. https://www.nejm.org/doi/full/10.1056/nejmoa1814052
2. https://www.nejm.org/doi/full/10.1056/NEJMoa1816885
3. https://www.sciencedirect.com/science/article/pii/S0735109719361807?via%3Dihub
4. https://www.fda.gov/news-events/press-announcements/fda-expands-indication-several-transcatheter-heart-valves-patients-low-risk-death-or-major
5. https://summitmd.com/html/technical/news_view.php?cmode=daily&subset=&page=6&no_case=97&part=c&year=
6. https://summitmd.com/html/technical/news_view.php?cmode=daily&subset=&page=1&no_case=137&part=t&year=
7. https://www.summitmd.com/html/technical/news_view.php?cmode=daily&subset=&page=4&no_case=83&part=c&year=
Transcatheter aortic valve replacement (TAVR) is expanding rapidly to younger and lower-risk aortic stenosis (AS) patients, becoming the therapy of choice in select subgroups and trumping even surgical aortic valve replacement (SAVR), an expert said.
 "At Asan Medical Center (AMC), the rule is that TAVR is the standard treatment when tissue valves are needed for symptomatic severe AS patients over 65 years of age," said Seung-jung Park, MD, PhD (AMC, Seoul, South Korea) at AP VALVES & STRUCTURAL HEART 2022 on Aug 11, which ran for two days at the Grand Walkerhill Seoul in South Korea.
"At Asan Medical Center (AMC), the rule is that TAVR is the standard treatment when tissue valves are needed for symptomatic severe AS patients over 65 years of age," said Seung-jung Park, MD, PhD (AMC, Seoul, South Korea) at AP VALVES & STRUCTURAL HEART 2022 on Aug 11, which ran for two days at the Grand Walkerhill Seoul in South Korea.
As chairman of AMC's Heart Institute, Park highlighted TAVR advances worldwide and at the medical institution over the past decade.

Major randomized controlled trials (RCTs) on TAVR and transcatheter heart valves (THVs) - including the CoreValve, Evolut and Sapien valves - laid the groundwork for device approval in both Europe and the US, ushering in the "era of TAVR" for patients who had no alternative to surgery.
TAVR has become comparable with surgery 5 to 8 years since its introduction and SAVR has become the alternative
The landmark PARTNER 31 and the Evolut Low Risk2 trials, published simultaneously in the New England Journal of Medicine (NEJM) in May 2019, backed the case for TAVR in lower operative risk groups after results favored TAVR over SAVR for the 30-day and 1-day endpoints of death, disabling stroke or rehospitalization.
Meta-analysis3 of the NOTION, SURTAVI, PARTNER 3 and Evolut Low Risk trials, published in the Journal of the American College of Cardiology (JACC) in Sep 2019, also showed 1-year outcomes favoring TAVR over SAVR for all-cause mortality (2.1% vs. 3.5%, RR 0.61; 95% CI, 0.39-0.96, I2=0%) and cardiovascular death (1.6% vs. 2.9%, RR 0.55, 0.33-0.90, p=0.02, I2=0%).
TAVR also had lower rates of disabling stroke (0.5 vs 1.7%), atrial fibrillation, life-threatening or disabling bleeding and acute kidney injury but higher rates of permanent pacemaker implantation and moderate or severe paravalvular leak (PVL).
On the heels of the major trials, the US Food and Drug Administration (FDA) greenlighted expanded indications for TAVR, for patients at low surgical risk in Aug 20194, causing seismic shifts in both American and European guidelines and boosting the once experimental procedure to a standard of care.
The 2020 American College of Cardiology and American Heart Association (ACC/AHA) guidelines gave a Class I recommendation for TAVR for patients over 65 years and the 2021 European Society of Congress and European Association of Cardio-Thoracic Surgeons (ESC/EACTS) guidelines greenlighted TAVR with a Class I indication for patients over 75 years5.
"More research is needed for TAVR in bicuspid aortic valve (BAV) disease, aortic and mitral bioprosthetic valve failure, low-flow, low-gradient AS, high-risk aortic regurgitation (AR) and routine use of cerebral protection devices, among others," he said.
"Nevertheless, TAVR has become comparable with surgery 5 to 8 years since its introduction, and SAVR has become the alternative."
AMC Celebrates 97% Procedural Success Rate During 22 Years of TAVR
 Following the first operation at AMC in 2010, - undertaken with TAVR pioneer Alain G. Cribier, MD(University of Rouen's Charles Nicolle Hospital, Paris, France),6 - TAVR operations at the South Korean hospital skyrocketed to over 250 cases per year.
Following the first operation at AMC in 2010, - undertaken with TAVR pioneer Alain G. Cribier, MD(University of Rouen's Charles Nicolle Hospital, Paris, France),6 - TAVR operations at the South Korean hospital skyrocketed to over 250 cases per year.
Last June, AMC's Heart Institute announced becoming the first in Asia to complete 1,000 TAVR cases7 and reported tallying 1,300 cumulative cases this year.
Analysis found AMC's Heart Team achieved a procedural success rate of 96.8% (1,101/1,137 patients) with low rates of new permanent pacemakers (6.9%) and moderate or severe paravalvular leaks (4.3%) despite operating on an elderly, high-risk and severe-disease population.
The rule at AMC, for tissue valves, is that TAVR has become the standard of treatment in patients with symptomatic severe AS patients over 65
Baseline characteristics showed the average patient was 80 years old, 47% were male and the mean Society of Thoracic Surgeons (STS) risk score was 4. Major comorbidities included hypertension (79.1%), diabetes (34.9%), chronic obstructive pulmonary disease (22%), atrial fibrillation (12.4%), stroke (11.7%) and peripheral vascular disease (5.1%).
Comparing 30-day and 1-year outcomes between cases from 2021 and all cumulative cases at AMC showed improvements in the endpoints of all-cause mortality (All cases 1.7% vs. 2021 cases 1.9%), and major, disabling strokes (1.0% vs. 0.3%).
TAVR also recorded improvements for the 30-day and 1-year endpoints of major vascular complications (5.3% vs. 0.0%), new permanent pacemaker implementation (6.9% vs. 3.0%) and moderate or severe PVR (4.3% vs. 1.1%) - which were aided by implementing a unique "minimalist approach."
"Since the first case in 2010, AMC developed a minimalist approach coined the 'MAC' that helps simplify the TAVR procedure," Park said. "Along with impeccable heart team collaboration, we also developed a meticulous pre-TAVR CT measurement algorithm that aids device selection.
The MAC approach implies no general anesthesia, no transesophageal echocardiography (TEE), 30-minute procedures, 1-day stays in the CCU, discharge within 3 days and a cardiac rehabilitation program, Park said.
Outcomes between balloon-expandable and self-expandable valves were also comparable, although self-expandable valves had a significantly higher rate of new permanent pacemakers (5.3% vs. 15.4%).
At AMC, the Edwards Lifesciences’ Sapien 3 valve (73%) accounted for most implants, followed by Sapien XT (Edwards Lifesciences; 10%), Evolut R & PRO (Medtronic; 8%), and CoreValve (Medtronic; 8%).
"Although valve durability remains a major issue and more research is needed on patient subgroups, outcomes at AMC have improved over time, thanks to MAC," Park said. "And the rule here, for tissue valves, is that TAVR has become the standard of treatment in patients with symptomatic severe AS patients over 65."
Edited by

Do-Yoon Kang, MD
Asan Medical Center, Korea (Republic of)
Written by

YoonJee Marian Chu, Medical Journalist
Read Biography2. https://www.nejm.org/doi/full/10.1056/NEJMoa1816885
3. https://www.sciencedirect.com/science/article/pii/S0735109719361807?via%3Dihub
4. https://www.fda.gov/news-events/press-announcements/fda-expands-indication-several-transcatheter-heart-valves-patients-low-risk-death-or-major
5. https://summitmd.com/html/technical/news_view.php?cmode=daily&subset=&page=6&no_case=97&part=c&year=
6. https://summitmd.com/html/technical/news_view.php?cmode=daily&subset=&page=1&no_case=137&part=t&year=
7. https://www.summitmd.com/html/technical/news_view.php?cmode=daily&subset=&page=4&no_case=83&part=c&year=


Leave a comment