News | AP VALVES & SH 2023
RENOVATE Trial
NOAC in Mechanical AVR: Is There a Hope?
The introduction of valve replacement surgery in the early 1960s dramatically improved the outcomes for patients with valvular heart disease. Currently, approximately 300,000 valve substitutes are implanted worldwide each year, with about half being mechanical valves and the other half bioprosthetic valves. This number is projected to rise to 850,000 per year by 2050.
Bioprosthetic valves pose a higher risk of reoperation due to structural valve deterioration, whereas mechanical valves typically require lifelong anticoagulation to prevent thromboembolism, leading to an increased risk of hemorrhage. The risk-benefit ratio between mechanical and bioprosthetic valves has led American and European guidelines on valvular heart disease to consistently recommend mechanical valves for patients under 60 years of age due to their more favorable long-term survival rates in younger patients. Despite these recommendations, the use of bioprosthetic valves has significantly increased across all age groups over the past decades, mainly due to the advantage of avoiding long-term anticoagulation.
In the current guidelines, oral anticoagulation using a vitamin K antagonist is recommended lifelong for all patients with mechanical valves. However, the use of vitamin K antagonists requires frequent blood testing, dosage adjustments, and restrictions on food, alcohol, and relevant drugs. To address these limitations, the Randomized, Phase II study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement (RE-ALIGN) trial was conducted. This trial aimed to compare the anticoagulation regimen between dabigatran and vitamin K antagonist(warfarin) in patients who had undergone mechanical heart valve replacement, evaluating the efficacy and safety of dabigatran, one of the non-vitamin K antagonist oral anticoagulants (NOACs), in patients with mechanical valves.
Unfortunately, the trial was terminated prematurely due to an excess of thromboembolic and bleeding events among patients in the dabigatran group. Consequently, the current guidelines contraindicate the use of NOACs in patients with mechanical valves. Despite this setback, revisiting the RE-ALIGN trial has provided valuable insights for future clinical trials. The major limitations of the RE-ALIGN trial include dose adjustments of dabigatran aiming for a trough plasma level of at least 50 ng/mL, often administering the drug above standard dosages, inclusion of mitral mechanical valve replacement cases (29%), which entail much higher thrombogenic conditions than aortic valve replacement, enrollment of patients in the early phase of the postoperative period (<3 months), known to be vulnerable to major bleeding, and the choice of a less potent Factor II inhibitor, dabigatran, instead of the more widely used Factor Xa inhibitors, which have proven higher efficacy in preventing thromboembolic events.
Considering these factors, we hypothesize that Factor Xa inhibitors have the potential to reduce the risk of bleeding complications after mechanical aortic valve replacement compared to conventional oral anticoagulation using vitamin K antagonists, especially in the late (<3 months) postoperative period. The RENOVATE trial is a multicenter, randomized, open-label, active-treatment, controlled trial designed to compare the safety of oral anticoagulation treatment with rivaroxaban and oral anticoagulation with vitamin K antagonist in patients at least 3 months after mechanical aortic valve replacement.
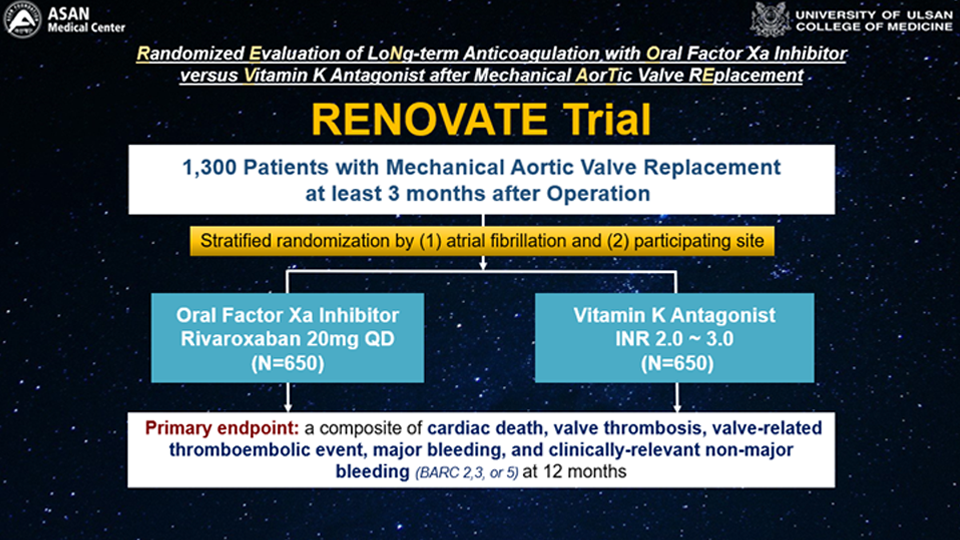
The primary endpoint of the study is a composite of cardiac death, valve thrombosis, valve-related thromboembolic events, major bleeding, and clinically relevant non-major bleeding [Bleeding Academic Research Consortium (BARC) 2, 3, or 5] at 12 months. The study is expected to have a total duration of about five years, including the preparation period, subject enrollment, and follow-up and analysis of results. Each participating institution will review the study through its clinical study committee. Target enrollment will begin after the approval of each clinical study committee and continue until 1300 subjects are enrolled. Consenting subjects will be randomized in a 1:1 ratio to either the rivaroxaban (NOAC) group, where Liroxia (rivaroxaban) 20mg is administered once daily after randomization, or the Vitamin K antagonist (warfarin) group, where Vitamin K antagonist (warfarin) will be taken with a target INR of 1.7~3.0 through planned INR monitoring after randomization. Randomization will be stratified based on the presence of atrial fibrillation and clinical site.
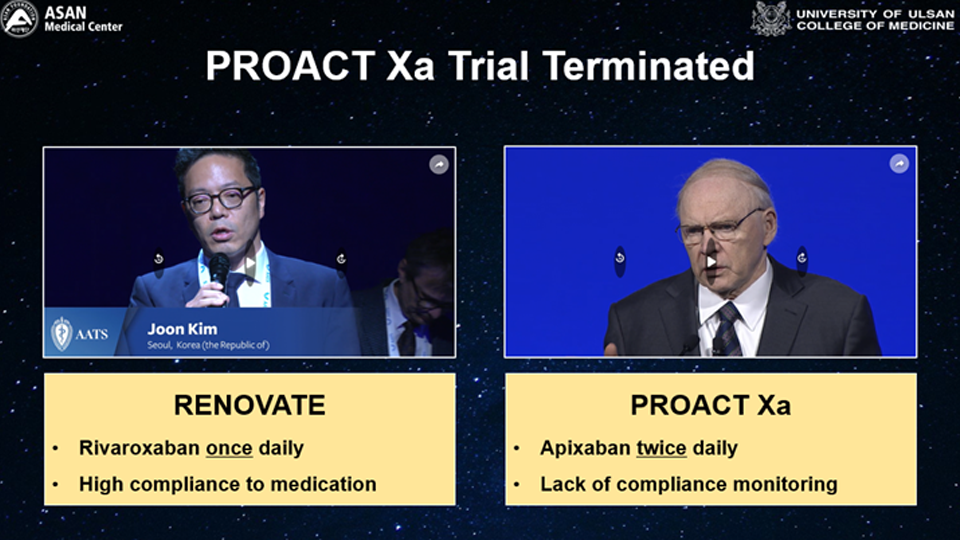
Another similar trial, the Prospective Randomized On-X Anticoagulation Clinical Trial (PROACT), recently presented its results after enrolling 863 participants. It demonstrated a significantly higher incidence rate of thromboembolism with NOAC (apixaban) compared to warfarin in individuals who had undergone On-X mechanical AVR. Based on these results, the Drug Safety Monitoring Board suggested interim analyses of the RENOVATE trial after enrolling 280 participants. The analysis showed no significant concerns, allowing the trial to proceed. Differences between the PROACT and RENOVATE trials include the use of different NOAC drugs (rivaroxaban vs. apixaban), different mechanical AVR devices (all mechanical AVR vs. On-X valve only), and differences in racial groups (Korean vs. mostly American white).

Ho Jin Kim, MD, PhD (Asan Medical Center) presents the RENOVATE Trial during the 12th AP VALVES & STRUCTURAL HEART 2023 held at the Grand Walkerhill Seoul, South Korea on August 11.
Looking forward to seeing exciting future
Our endeavor is to redefine the landscape of anticoagulation strategies, making decisions more patient-centric and enhancing the quality of life for patients who undergo mechanical AVR. We remain hopeful about the potential use of NOACs in low-risk patients who have undergone mechanical AVR, which may counterbalance the increasing use of bioprosthetic AVR, including TAVR, in the future. Anticipating further developments, we look forward to the RENOVATE trial sharing its final results within the next five years.

The Trial’s Principal Investigator, Joon Bum Kim, MD, PhD (Asan Medical Center, Seoul, South Korea) also presented the RENOVATE Trial at the Heart Valve Disease Forum 2023 at the Seoul Dragon City in South Korea on Sep 16.
Edited by

Joon Bum Kim, MD
Asan Medical Center, Korea (Republic of)

KyungAe Kim, RN
CardioVascular Research Foundation (CVRF), Korea (Republic of)
The introduction of valve replacement surgery in the early 1960s dramatically improved the outcomes for patients with valvular heart disease. Currently, approximately 300,000 valve substitutes are implanted worldwide each year, with about half being mechanical valves and the other half bioprosthetic valves. This number is projected to rise to 850,000 per year by 2050.
Bioprosthetic valves pose a higher risk of reoperation due to structural valve deterioration, whereas mechanical valves typically require lifelong anticoagulation to prevent thromboembolism, leading to an increased risk of hemorrhage. The risk-benefit ratio between mechanical and bioprosthetic valves has led American and European guidelines on valvular heart disease to consistently recommend mechanical valves for patients under 60 years of age due to their more favorable long-term survival rates in younger patients. Despite these recommendations, the use of bioprosthetic valves has significantly increased across all age groups over the past decades, mainly due to the advantage of avoiding long-term anticoagulation.
In the current guidelines, oral anticoagulation using a vitamin K antagonist is recommended lifelong for all patients with mechanical valves. However, the use of vitamin K antagonists requires frequent blood testing, dosage adjustments, and restrictions on food, alcohol, and relevant drugs. To address these limitations, the Randomized, Phase II study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement (RE-ALIGN) trial was conducted. This trial aimed to compare the anticoagulation regimen between dabigatran and vitamin K antagonist(warfarin) in patients who had undergone mechanical heart valve replacement, evaluating the efficacy and safety of dabigatran, one of the non-vitamin K antagonist oral anticoagulants (NOACs), in patients with mechanical valves.
Unfortunately, the trial was terminated prematurely due to an excess of thromboembolic and bleeding events among patients in the dabigatran group. Consequently, the current guidelines contraindicate the use of NOACs in patients with mechanical valves. Despite this setback, revisiting the RE-ALIGN trial has provided valuable insights for future clinical trials. The major limitations of the RE-ALIGN trial include dose adjustments of dabigatran aiming for a trough plasma level of at least 50 ng/mL, often administering the drug above standard dosages, inclusion of mitral mechanical valve replacement cases (29%), which entail much higher thrombogenic conditions than aortic valve replacement, enrollment of patients in the early phase of the postoperative period (<3 months), known to be vulnerable to major bleeding, and the choice of a less potent Factor II inhibitor, dabigatran, instead of the more widely used Factor Xa inhibitors, which have proven higher efficacy in preventing thromboembolic events.
Considering these factors, we hypothesize that Factor Xa inhibitors have the potential to reduce the risk of bleeding complications after mechanical aortic valve replacement compared to conventional oral anticoagulation using vitamin K antagonists, especially in the late (<3 months) postoperative period. The RENOVATE trial is a multicenter, randomized, open-label, active-treatment, controlled trial designed to compare the safety of oral anticoagulation treatment with rivaroxaban and oral anticoagulation with vitamin K antagonist in patients at least 3 months after mechanical aortic valve replacement.

The primary endpoint of the study is a composite of cardiac death, valve thrombosis, valve-related thromboembolic events, major bleeding, and clinically relevant non-major bleeding [Bleeding Academic Research Consortium (BARC) 2, 3, or 5] at 12 months. The study is expected to have a total duration of about five years, including the preparation period, subject enrollment, and follow-up and analysis of results. Each participating institution will review the study through its clinical study committee. Target enrollment will begin after the approval of each clinical study committee and continue until 1300 subjects are enrolled. Consenting subjects will be randomized in a 1:1 ratio to either the rivaroxaban (NOAC) group, where Liroxia (rivaroxaban) 20mg is administered once daily after randomization, or the Vitamin K antagonist (warfarin) group, where Vitamin K antagonist (warfarin) will be taken with a target INR of 1.7~3.0 through planned INR monitoring after randomization. Randomization will be stratified based on the presence of atrial fibrillation and clinical site.

Another similar trial, the Prospective Randomized On-X Anticoagulation Clinical Trial (PROACT), recently presented its results after enrolling 863 participants. It demonstrated a significantly higher incidence rate of thromboembolism with NOAC (apixaban) compared to warfarin in individuals who had undergone On-X mechanical AVR. Based on these results, the Drug Safety Monitoring Board suggested interim analyses of the RENOVATE trial after enrolling 280 participants. The analysis showed no significant concerns, allowing the trial to proceed. Differences between the PROACT and RENOVATE trials include the use of different NOAC drugs (rivaroxaban vs. apixaban), different mechanical AVR devices (all mechanical AVR vs. On-X valve only), and differences in racial groups (Korean vs. mostly American white).

Ho Jin Kim, MD, PhD (Asan Medical Center) presents the RENOVATE Trial during the 12th AP VALVES & STRUCTURAL HEART 2023 held at the Grand Walkerhill Seoul, South Korea on August 11.
Looking forward to seeing exciting future
Our endeavor is to redefine the landscape of anticoagulation strategies, making decisions more patient-centric and enhancing the quality of life for patients who undergo mechanical AVR. We remain hopeful about the potential use of NOACs in low-risk patients who have undergone mechanical AVR, which may counterbalance the increasing use of bioprosthetic AVR, including TAVR, in the future. Anticipating further developments, we look forward to the RENOVATE trial sharing its final results within the next five years.

The Trial’s Principal Investigator, Joon Bum Kim, MD, PhD (Asan Medical Center, Seoul, South Korea) also presented the RENOVATE Trial at the Heart Valve Disease Forum 2023 at the Seoul Dragon City in South Korea on Sep 16.
Edited by

Joon Bum Kim, MD
Asan Medical Center, Korea (Republic of)

KyungAe Kim, RN
CardioVascular Research Foundation (CVRF), Korea (Republic of)

Leave a comment